Corrositex® OECD 435
Home » InVitro International » Corrositex
The 100% animal free OECD skin corrosion test
Corrositex® OECD TG 435 identifies corrosive chemical substances and mixtures (UN GHS Cat.1) and their sub-category 1A, 1B and 1C by means of an artificial membrane designed to respond to corrosive substances in a manner similar to animal skin in situ.
- Globally accepted and recognized
- Results in as little as 3 minutes and no more than 4 hours
- Easy to use
- 100% animal free
- No need for a cell biology or cellular coluture lab
- Long storage period
- Easily stored
The only one able to identify sub-categories 1A, 1B and 1C
TG OECD 431 only allows discrimination between Sub-cat. 1A from those 1B and 1C.
TG OECD 430 is unable to discern between any subcategories.
Only Corrositex® OECD 435, among all alternative in vitro methods OECD adopted, is able to identify and classify the Cat.1 sub-category of the test substance.
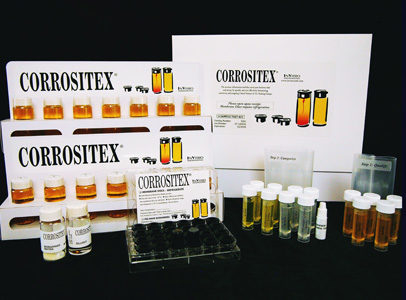
The Assay System
The Corrositex® Assay System consists of a glass vial filled with a chemical detection fluid coated with a proprietary membrane that acts as a bio-barrier, the system is designed to mimic the effect of corrosives on living skin.
How does it work?
Corrositex® is performed in three steps:
1. First, a qualification test is performed to ensure that the test sample and CDS reagent are compatible. If a physical change or color change is observed, the sample is judged compatible with the detection solution and the rest of the test is performed.
2. The second phase of the Corrositex® test uses appropriate indicator solutions to allow categorization of the test sample as Corrositex® Category 1 or Corrositex® Category 2 material.
Corrositex® Category 1 materials are typically strong acids/bases, while Corrositex® Category 2 materials are typically weak acids/bases.
3.The third step of the test is performed by applying the sample to the bio-barrier. When the chemical penetrates through or destroys the entire thickness of this biological barrier, it comes into contact with the CDS which then undergoes a simple colour change.
This colour change is observed visually and the time required for the colour change to occur is recorded.
Once you have registered the time that the sample needs to perforate the membrane you can assign the correct GHS category and/or U.N. packaging group classification for U.S. DOT or EPA compliance, or use the data as a classification tool or to validate marketing claims.
Why choose Corrositex®?
Because it will save you time and money:
- Unlike animal testing which can take 2 to 4 weeks, Corrositex® tests can provide a determination of the packing group in as little as 3 minutes and no more than 4 hours.
- Many in vitro skin corrosivity tests, such as OECD 431, are only able to discriminate between sub-category 1A and sub-categories 1B and 1C. Corrositex® is able to identifyto which sub-category the sample belongs.
- Users of the Corrositex® method save in shipping, testing and running costs for each individual sample tested.
Declassify your product with Corrositex® and Flashpoint
Acquire objective evidence regarding a classification or declassification for the possible corrosive hazard of your tested sample.
Are you a Regulatory affairs manager, REACH /CLP manager, Laboratory manager, Dangerous goods expert?
With Corrositex you will be able to manage in full autonomy the determination of the corrosiveness of your formulations, saving time and costs for the contract to an external laboratory, and you will be able to possibly give a less dangerous classification to its products in full compliance with current legislation.
Attend the next “Practical course for the determination of chemical corrosivity with Corrositex” by our partner Flashpoint srl, one of the most authoritative European consulting and training companies for the management and transport of substances, mixtures, articles and hazardous waste.
Classify your waste with Corrositex®.
For wastes whose composition is unknown or cannot be determined, the HP 8 ((Corrosive) can be determined, according to the approach inferred from the CLP for mixtures, by application of the test methods described in the regulation 440/20083 or other internationally recognized methods.
Corrositex® is authorized for this use and is reported in the same regulation 440/20083 at paragraph B.46.
Corrositex® is also mentioned in Table R.7.2-2 “Accepted in vitro test methods for skin corrosion/irritation” of the ECHA guideline “Chapter R.7a: Endpoint specific guidance Version 6.0 – July 2017” for fulfilling obligations under REACH.
In addition, European Commission Regulation (EU) 2017/735 of February 14, 2017 amends Regulation (EC) no. 440/2008 and refers explicitly (first paragraph of the “Initial Considerations“) to our OECD, Test No. 435: In vitro Membrane Barrier Test Method for Skin corrosion (Corrositex®).

Material Safety Data Sheet and Regulatory Acceptance
United Nations (UN) GHS
Acceptance, Globally Harmonised System of Classification and Labelling of Chemicals (GHS). Fifth revised edition, UN New York and Geneva, 2013. P. 126 (d).
EU / OECD
Approval of 19 July 2006
European Centre for the Validation of Alternative Methods (ECVAM)
12/2002
US Department of Transportation – DOT-E 10904
Original exemption granted on 28 April 1993
US DOT (PHMSA) communication that eliminates the need for SP-10904
Transport Canada – Authorisation for an equivalent level of safety SU 4483
Original approval 14/8/96
Additional renewal 18/09/98
Additional renewal 15/01/01
Acceptance of Draize Full 3/5/02 replacement
US Occupational Safety and Health Administration (OSHA)
Letter of interpretation of 3 March 1994
Formal acceptance, NIEHS press release of 21/03/00
International Air Transport Association (IATA)
Letter of acceptance of 17 December 1993
US Federal Register EPA / Vol. 60, no. 142 Skin corrosion method 1120 13 June 1997
Formal acceptance, NIEHS press release of 21/03/00
Committee on Consumer Product Safety (CPSC)
Formal acceptance, NIEHS press release of 21/03/00