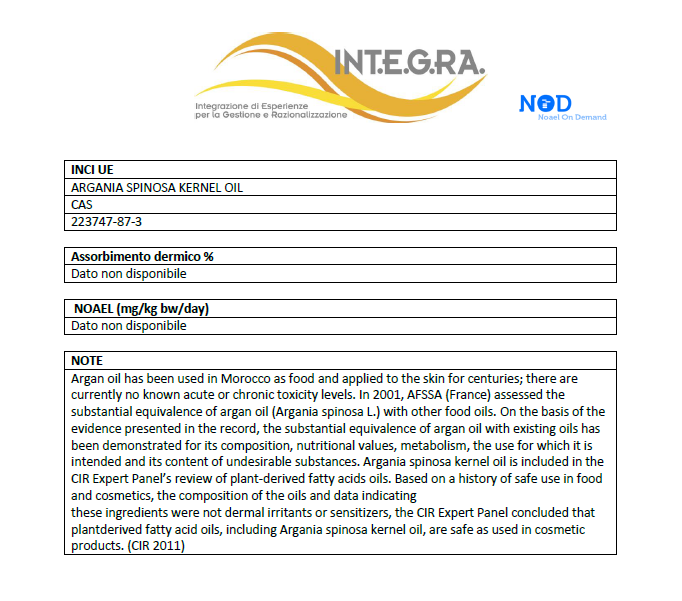
The NOAEL database for your needs
NoD (NOAEL On Demand) is a database of toxicological data, the NOAEL (No Observed Adverse Effect Level), created in response to the need of regulatory managers in the cosmetics industry to have pre-processed, pre-filtered and pre-selected information available.
The database includes over 800 substances and is in constant growth.
The NOAEL in the Product Information File
In order to release a cosmetic product on the market, it is necessary to fill in the PIF, whichmust take NOAELs into account when calculating the MoS (Margin of Safety).
MoS is considered and calculated according to the safety of certain substances used in the formulation of a cosmetic product. The Mos of a substance can be calculated by dividing its NOAEL value, chosen and motivated by the Safety Assessor, by its possible SED, as described in “SCCP’s notes of guidance for the testing of cosmetic ingredients and their safety evaluation”.

How does it work?
The datasheets
Once the quotation is confirmed, the data sheetswill be provided in a protected .pdf format.
For Dossier Manager® users, the sheets will be automatically attached in the BIP chapters that contain the substances they refer to, and the toxicological data contained will be automatically recorded in the program for MOS calculation.
In case no NOAEL data is available for an ingredient, a summary note on the different aspects related to the dermal effects of the ingredient itself published by authoritative sources is provided, providing useful information for the safety assessor. On the basis of the available data, a summary assessment of the safety of the ingredient in cosmetic products shall be carried out whenever possible.
New! DB TOX Dossier Manager
Are you a Dossier Manager user and need more than a few toxicological monographies? Subscribe to DB TOX, the new module to get toxicology references for more than 650 INCIs directly into your Manager.
- Download the entire catalogue
- Check the INCI you need
- Send us the excel worksheet to segreteria.commerciale@integracosmetics.com
Note on selection criteria and information collection
The No Observed Adverse Effect Level (NOAEL) – dose without observable adverse effect – is defined as the highest dose or level of exposure, for which no treatment-related adverse effects are observed. The NOAEL of a substance is derived from Repeated Dose Toxicity studies, which involve the daily administration of predefined doses of the substance for prolonged periods of time. NOAEL is expressed in mg/kg bw/day.
For the determination of NOAEL of a substance, only studies published by reliable sources (e.g. ECHA, CIR, SCCS, EFSA, toxicological databases, scientific literature) are considered. In the absence of such studies, the value reported by the supplier of the raw material, if available, may be taken into account.
The available repeated dose toxicity studies are analyzed in detail to evaluate the type of study conducted, the method of administration, the dosages, the toxicological effects monitored, their severity and reversibility.
As a general principle, if different NOAEL values are available for the same substance, you prefer to choose the more conservative (lower) value. More specifically, the following selection criteria are applied:
– Studies in which multiple doses have been tested and, among these, those in which NOAEL does not correspond to the maximum tested dose are preferred;
– studies conducted on rodents, preferably rats, are preferred in the following order: subchronic toxicity (90 days), chronic (2 years), carcinogenesis, developmental and reproductive toxicity, short-term toxicity (28 days).
The information published according to these criteria has been collected and proposed in good faith; this does not relieve the Safety Assessor from its responsibilities under the Standard.