Irritection® + Corrositex® Protocols
Home » InVitro International » Protocols
Ocular Irritection®
OECD Test Guideline No. 496
In vitro Macromolecular Test Method for Identifying Chemicals Inducing Serious Eye Damage and Chemicals not Requiring Classification for Eye Irritation or Serious Eye Damage

Irritection® Assay System
Ocular Irritection OECD TG 496
Assay Protocol
OECD TG 496: In vitro Macromolecular Test Method for Identifying Chemicals Inducing Serious Eye Damage and Chemicals not Requiring Classification for Eye Irritation or Serious Eye Damage
DB-ALM Protocol n° 157 : Ocular Irritection® Assay System
This assay enables to assess the potential of a test compound to cause eye irritancy and corneal opacityby measuring the increase in optical density produced by the interaction of the test material with aprotein matrix. The assay is currently available as a kit comprised of reagents and computer softwarethat have been integrated with the instrumentation in the testing laboratory to provide an automated invitro test.
Irritection® Assay System
Instruction Manual
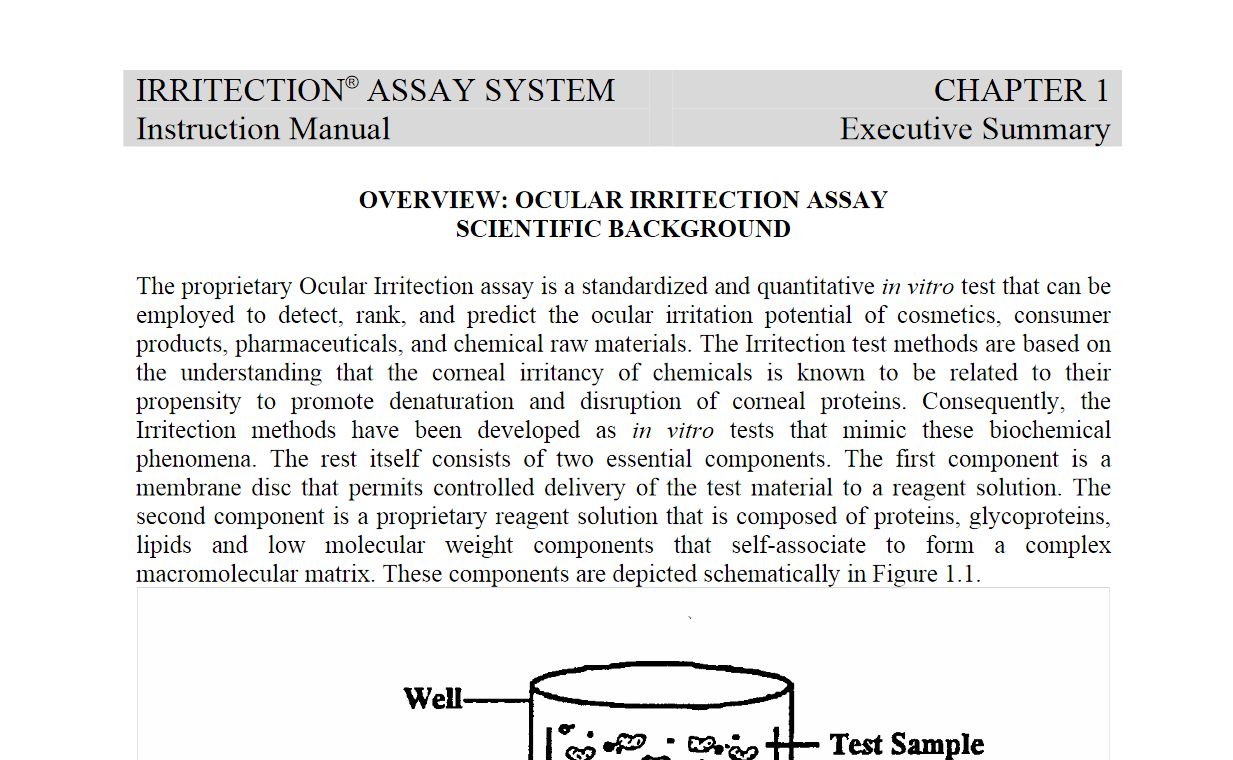
The proprietary Ocular Irritection assay is a standardized and quantitative in vitro test that can be employed to detect, rank, and predict the ocular irritation potential of cosmetics, consumer products, pharmaceuticals, and chemical raw materials. The Irritection test methods are based on the understanding that the corneal irritancy of chemicals is known to be related to their propensity to promote denaturation and disruption of corneal proteins.
Irritection® Dermal
Irritection® Assay System
Dermal Response Assay Protocol
In Vitro International’s Dermal Response Assay is an in vitro test which utilizes changes ofrelevant macromolecules topredict the dermal irritation potential of surfactant and non–surfactant based chemical, petrochemical, and cosmetic samples. The specially engineered Dermal Bio–barrier closely duplicates the normal barrier propertiesof the dermis.
When the test is performed, the changes in protein structure that are induced by the test material are then quantified by measuring the resulting changes in the turbidity (optical density) of the reagent solution at a wavelength of 450 nm using a spectrometer.
Corrositex®
Corrositex® Assay System
Reference and instruction manual
Skin corrosion refers to the production of irreversible damage to the skin, manifested as visible necrosis through the epidermis and into the dermis, following the application of a test chemical as defined by the United Nations (UN) Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (1). This updated Test Guideline 435 provides an in vitromembrane barrier test method that can be used to identify corrosive chemicals. The test method utilizes an artificial membrane designed to respond to corrosive chemicals in a manner similar to animal skin in situ.
OECD Test Guideline No. 435
This updated Test Guideline 435 provides an in vitro membrane barrier test method that can be used to identify corrosive chemicals. The test method utilizes an artificial membrane designed to respond to corrosive chemicals in a manner similar to animal skin in situ.